



*No Daily end-of-day manual replacement and compounding of water solution
SoftWave offers providers confidence in safety and effectiveness with four FDA Clearances and an FDA-inspected U.S. facility. Stemwave is only FDA-registered with NO FDA Clearances and is not FDA inspected.
Founded in 2004, SoftWave technology is widely used and endorsed by experts in orthopedics, wound care, and urology. It is utilized at leading medical centers like Harvard, UCLA, and Cleveland Clinic, and trusted by major sports organizations, including MLB, NFL, NBA, and UFC, to treat professional athletes.
SoftWave has a flexible applicator with a clear membrane allowing the provider to see if there are any gas bubbles. (Gas bubbles prevent the energy from reaching the target area.) Stemwave does not have a clear membrane.
SoftWave’s certified trainers provide on-site training, eliminating disruptions to your everyday operations. Our training days double as real patient sessions, turning education into a revenue-generating opportunity for your practice. In addition, we save your clinic time and resources by eliminating the need for off-site travel and accommodation.
SoftWave has over 1400 clinics in North America and the Provider Search on the www.softwavetrt.com website allows prospective patients to easily locate your practice.
Modus-F does not provide a national provider search.
SoftWave’s patented parabolic reflector produces a broad focused wave providing a deeper and wider column of acoustic energy utilizing the physics of having a primary and secondary wave of energy for better outcomes.
Modus-F uses focused technology.
SoftWave has two decades of use in the U.S. and has spent $25 million on research and development. There are 24o papers from 98 universities globally. It is endorsed and utilized by eminent Key Opinion Leaders in orthopedics, wound care, urology, and more, as well as from national sports teams and leading academic institutions such as Harvard, UCLA, Cleveland Clinic, etc. It has 47 patents plus patents pending as well as 30+ active clinical trials and studies.
SoftWave uses a continuous loop water system fed by a contained water cartridge. This ensures the proper water filtration as each spark produces a gas.
SoftWave has a specialized service department with highly trained nationally certified technicians.
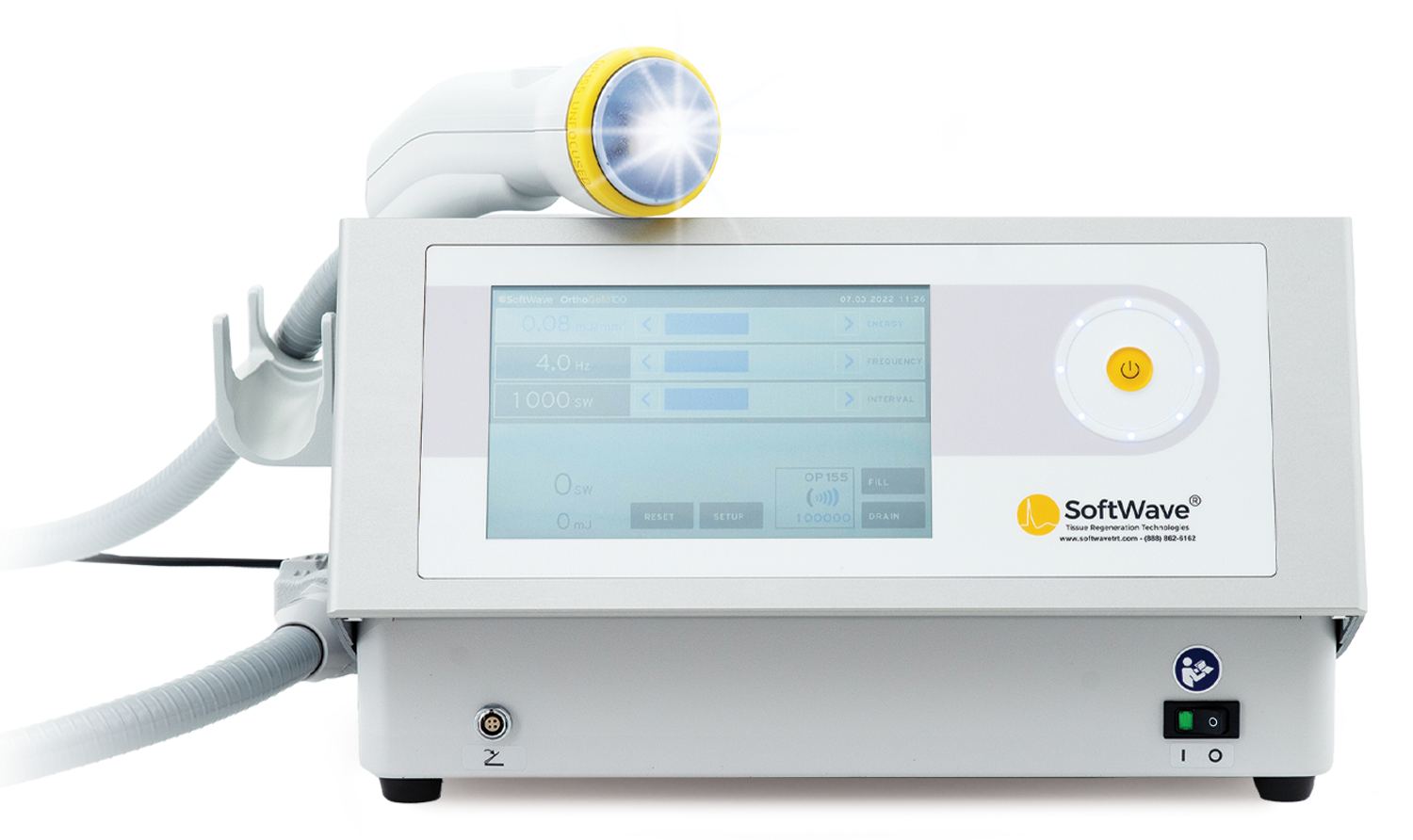
SoftWave’s applicator is both lighter and ergonomically designed for comfort. In times of staffing challenges and training costs, SoftWave prioritizes ease of use to retain and support your invaluable team.
SoftWave’s energy, delivered through a parabolic reflector, penetrates wide and deep, ensuring patient comfort and efficacy with fewer pulses.
SoftWave can sit on a tabletop and easily integrate into your space.

Stemwave Modus-F does not have a water filtration system which means the technician must manually fill the water in the applicator head and manually eliminate the air bubbles as they happen. This happens during treatments which interrupts care and add time for everyone.
With the Modus-F, the responsibility is on the user to change their own electrodes – mixing electricity and water. Because of this, the Modus-F applicator rusts after a short period of time. This compromises aesthetics and sterility, vital in a healthcare setting.
The Modus-F applicator is heavier and more cumbersome which is added stress to the user.
Modus-F’s focused energy delivery can cause more discomfort and potentially less successful tissue targeting due to its converging point of release.
Modus-F is one unit measuring 3 feet high.