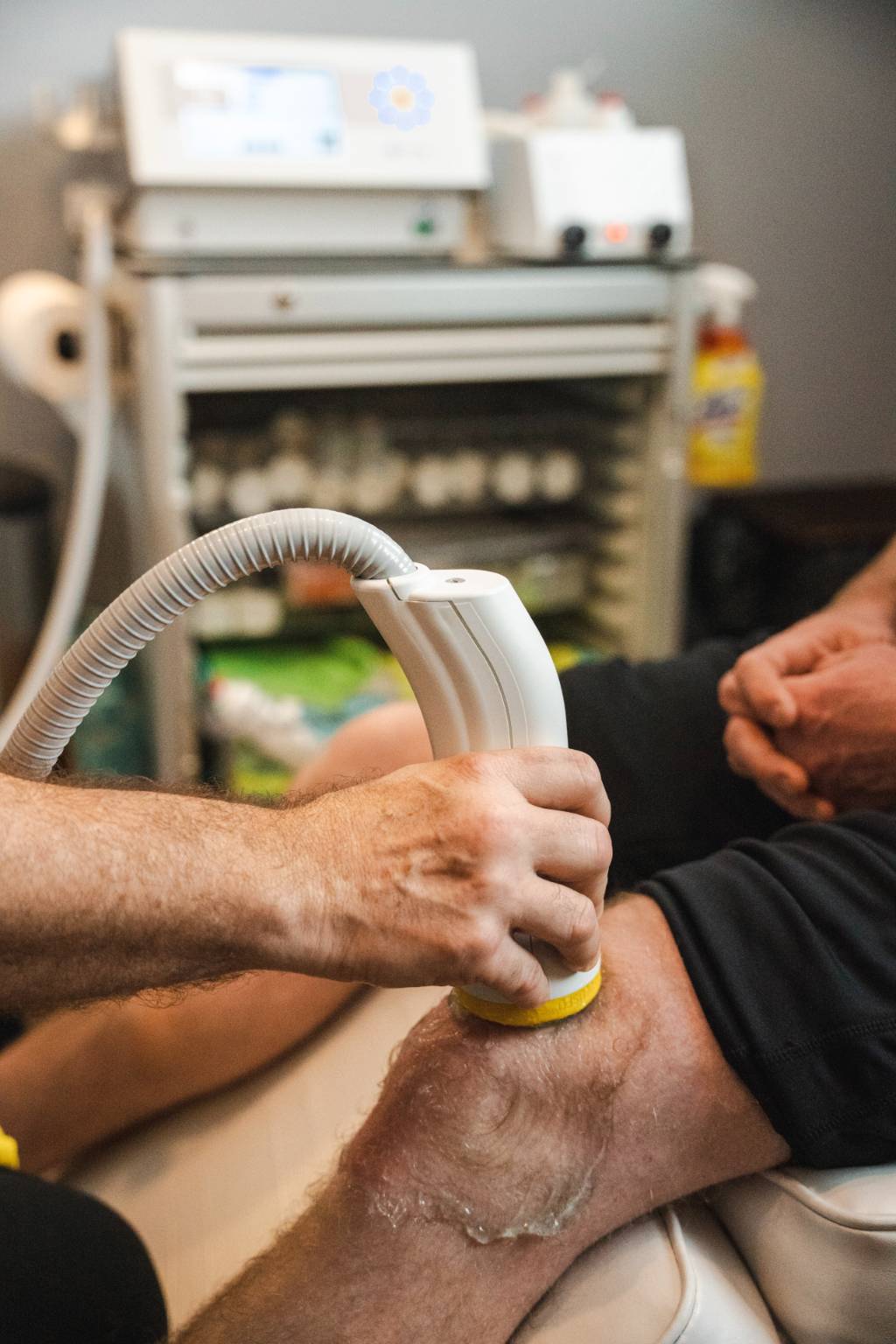
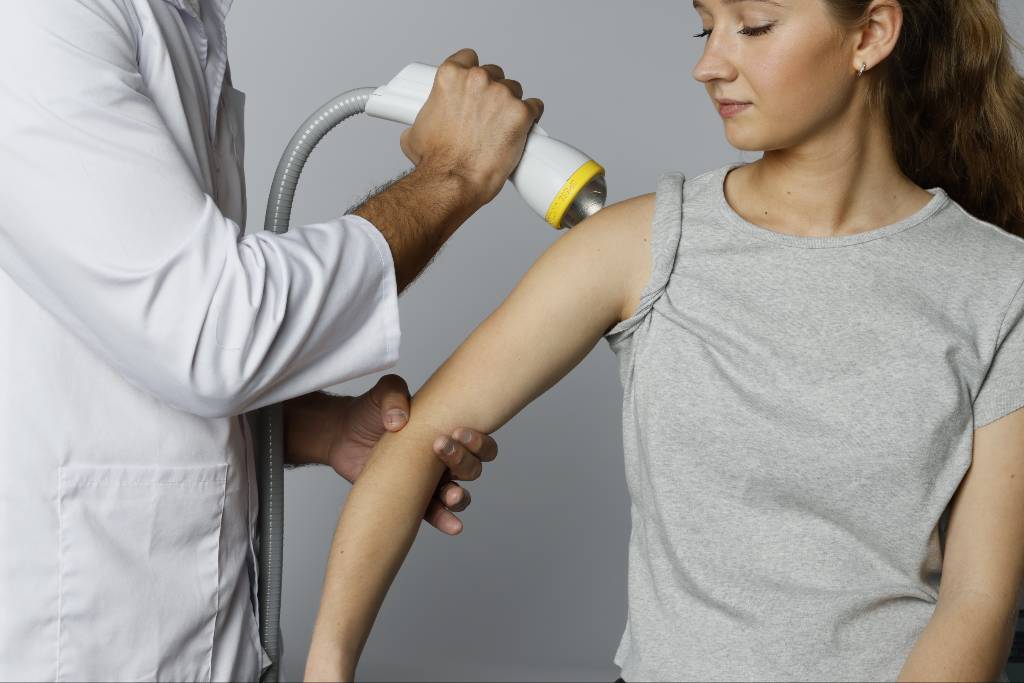
Shockwave therapy has become an evidence-based approach for stimulating tissue repair and improving pain outcomes across orthopedics, physical medicine, and regenerative medicine. As more shockwave medical devices enter the market, differences in technology and clinical validation have created uncertainty among providers. Some systems marketed as shockwave technology produce only surface-level mechanical pulses with limited depth and reproducibility, which may compromise outcomes.
For clinicians comparing regenerative technologies, knowing which questions to ask helps distinguish proven medical systems from those driven primarily by marketing language. Selecting the right device is a matter of patient safety, clinical efficacy, and long-term return on investment.
10 Questions Providers Should Ask Before Choosing a Shockwave Device
For clinicians evaluating regenerative technologies, the decision to invest in a shockwave medical device carries clinical and operational importance. The following considerations help identify which systems are engineered for consistent performance, patient comfort, and reproducible outcomes.
1. Is the specific Device FDA Class II Cleared or at Least Class I?
FDA clearance defines whether a device is approved for legitimate medical use. A Class II clearance confirms that the device has been reviewed for both safety and effectiveness for specific clinical indications.
In contrast, Class I registration applies to low-risk products such as therapeutic massagers, which are not designed for medical outcomes beyond temporary pain relief. Clinicians should always verify a manufacturer’s 510(k) number and confirm that the clearance aligns with their intended applications.
Ask the salesperson to send you the 510(k) registration for their device.
2. Does It Generate True Shockwaves or Only Radial Pressure Waves?
True shockwaves are supersonic mechanical impulses that stimulate biological repair at depth. They can be generated through electrohydraulic, electromagnetic, or piezoelectric technologies, each varying in energy distribution and treatment precision. Broad focused electrohydraulic systems (Softwave) produce a strong, spherical wave that activates a larger tissue volume, while focused only electrohydraulic (Stemwave, Omniwave), electromagnetic (Stortz, Chattanooga) and piezoelectric devices (Piezowave) create narrower focal zones requiring more precise targeting.
Radial or acoustic pressure waves are slower, surface-level pulses that lack the depth and rise time of true shockwaves, reducing their regenerative potential and treatment consistency.
3. How Broad Is the Treatment Zone and Does It Activate More Tissue With Fewer Sessions?
The treatment zone determines how much tissue volume is affected per pulse. Broad-focused systems activate a larger biologic area, which allows for fewer shocks and shorter treatment sessions. Devices with narrow focal zones require pinpoint precision, and along with radial pressure wave devices often lead to higher discomfort and extended treatment times. Reviewing a manufacturer’s technical specifications on energy depth and spread helps clinicians compare therapeutic efficiency between systems.
4. What Published Research or Registry Data Support This Device?
Peer-reviewed publications, inclusion in medical guidelines, and independently collected registry data demonstrate that outcomes have been validated scientifically. A lack of device-specific data raises questions about reproducibility and safety. Providers should request references or published data directly from the manufacturer and confirm them through recognized databases such as PubMed.
5. What Is the True Cost per Treatment, Including Maintenance and Labor?
The actual cost of operating a shockwave system extends beyond its initial purchase price. Factors such as treatment time, consumables, maintenance requirements, and expected session volume directly influence the cost per treatment.
Devices that require frequent servicing or high shock counts per session increase operational expenses including labor. Systems engineered for efficiency, durability, and minimal consumables provide a stronger long-term return on investment and allow clinics to maintain predictable margins.
6. Is On-Site Training Provided for the Entire Clinical Team?
Comprehensive training ensures consistent outcomes and patient safety. High-quality vendors provide on-site onboarding by experienced professionals, often clinicians, that covers both operation and clinical application, equipping staff with indication-specific protocols and best practices. When training is limited to a basic manual and device handling or requires additional travel costs, adoption and effectiveness suffer. Consistent followup education supports provider confidence, proper dosing, and optimal patient experience.
7. Is There a U.S.-Based Service Center, and How Simple Is Daily Upkeep?
Maintenance reliability influences both safety and clinic workflow. Systems that require daily water changes, manual degassing, or specialized tools for part replacement, and changing of heads to reach different depths of biological effect add unnecessary complexity and downtime. A well-designed device incorporates a closed-loop water system, automatic degassing, and simple component replacement to ensure continuous, safe use. Access to a U.S.-based service center also reduces turnaround time for support and maintenance.
8. How Long Has the Company Been Established and What Patents Support Its Technology?
A manufacturer’s history and intellectual property portfolio reflect its credibility and commitment to innovation. Clinicians should review how long the company has been producing medical devices and whether it holds patents for unique engineering features such as reflector geometry or electrode design. Patented technology demonstrates original research and reliable development, whereas unverified imports may lack performance consistency and long-term support.
9. Is Treatment Comfortable, Well Tolerated, and Guided by Real-Time Feedback?
Patient comfort determines compliance and overall satisfaction with therapy. Systems that distribute energy broadly with a focused component and maintain consistent intensity tend to produce less discomfort than those with narrow focal zones only. Real-time feedback allows clinicians to identify reactive or symptomatic regions during treatment, improving precision and patient trust. These elements contribute directly to treatment success and practice reputation.
10. Who Is Using This Technology in Leading Clinics or Professional Organizations?
Adoption among respected medical institutions is one of the strongest indicators of reliability. Devices utilized in academic hospitals, rehabilitation centers, and professional sports organizations have generally undergone rigorous evaluation. Providers should confirm whether the system is trusted in environments where performance, consistency, and safety are non-negotiable. A well-established user base offers confidence in both outcomes and long-term manufacturer support.
For guidance on making evidence-based choices when comparing systems, consult The Clinician’s Guide to Shockwave Therapy: Evidence, Safety, and Technology.
How to Evaluate Your Shockwave Medical Device Answers
Comparing responses across manufacturers is most effective when organized into a structured chart or data table. A clear visual framework enables clinicians to compare the scientific validity, operational performance, and overall practicality of each shockwave medical device side by side.
Create a comparative table with key evaluation categories that correspond to the questions already reviewed:
- Regulatory Status
- Technology Type
- Treatment Zone
- Clinical Evidence
- Operational Cost
- Training and Support
- Company Record and Innovation
- Service and Maintenance
- Patient Experience
- Clinical Adoption
Analyzing devices through a comparison grid helps remove subjective bias and marketing influence. The most credible systems stand out through quantifiable performance, transparent evidence, and reproducible outcomes.
SoftWave Therapy: The Evidence-Based Advancement for Regenerative Medicine
Selecting the right shockwave medical device requires alignment between scientific proof, engineering precision, and clinical reliability. SoftWave Therapy fulfills all three through patented technology and verified outcomes that reflect its role as the trusted choice among regenerative medicine providers.
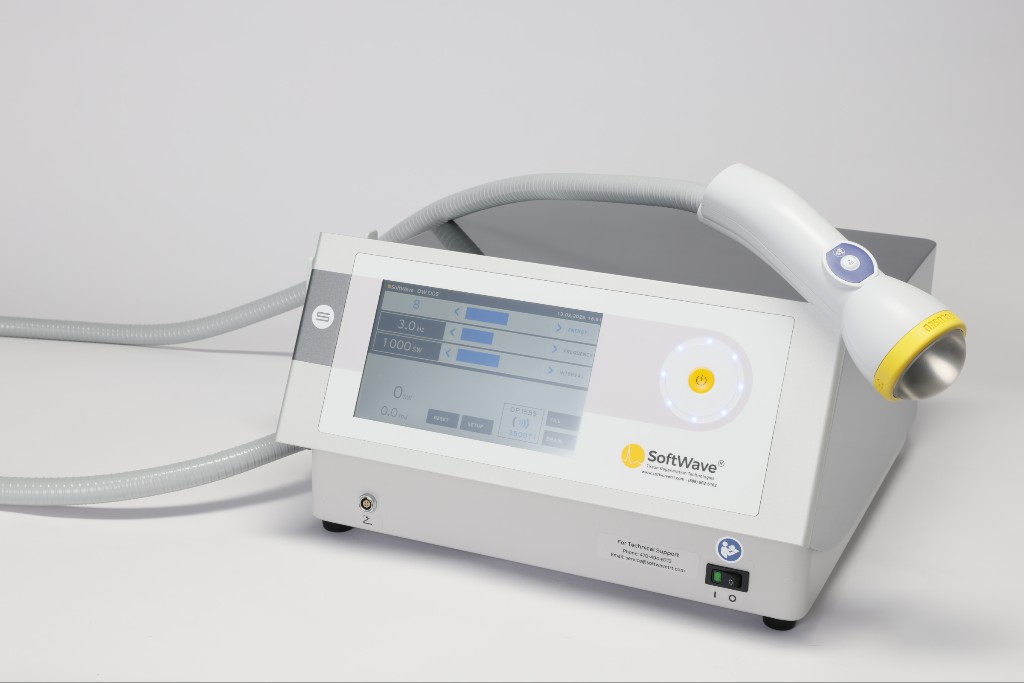
Patented Broad-Focused Electrohydraulic Technology
SoftWave Therapy uses a patented electrohydraulic design that produces true broad-focused shockwaves. Each pulse is generated through plasma discharge in water, creating a spherical wave that reaches both superficial and deep tissue. The parabolic reflector forms a 7 cm by 12 cm field, activating more tissue with fewer shocks without causing microtrauma or discomfort.
FDA-Cleared for Multiple Regenerative Indications
SoftWave Therapy holds several FDA 510(k) clearances, including activation of connective tissue, enhancement of local blood circulation, treatment of acute second-degree burns, and chronic diabetic foot ulcers. These clearances verify its safety and effectiveness for regenerative use and differentiate it from devices limited to temporary pain relief claims.
Engineered for Clinical Efficiency and Safety
Built for reliability, SoftWave features a closed-loop water system that removes manual refills, auto-degas technology for consistent wave quality, and SmartTrode® electrodes that self-adjust to maintain waveform precision. These features minimize downtime, streamline setup, and ensure uniform energy delivery across treatments.
Proven Through Research and Real-World Clinical Use
SoftWave’s electrohydraulic design is supported by an expanding body of peer-reviewed research across orthopedics, sports medicine, physical therapy, urology, and podiatry. Studies demonstrate its ability to promote angiogenesis, modulate inflammation, and accelerate tissue regeneration. Its use at Mayo Clinic, UCLA, Baylor, Cleveland Clinic, and within professional sports organizations reinforces its reliability under clinical scrutiny.
Comprehensive Training and U.S.-Based Support
Every SoftWave system includes on-site onboarding, access to online education, and continuous protocol updates. A U.S.-based service center manages maintenance and technical support, ensuring uninterrupted clinical operation and confidence for providers.
If you are evaluating technology for your practice, learn more about the Best Shockwave Therapy Machine for Providers.
Take the Next Step Toward Better Patient Outcomes With SoftWave Therapy
The process of selecting a shockwave medical device depends on evidence, not marketing language. Evaluating technology through measurable factors ensures that the system chosen supports real biological healing and predictable outcomes. The right equipment improves patient results and strengthens professional trust and long-term clinical success.
SoftWave Therapy represents the next generation of shockwave innovation, built for reproducibility, safety, and confidence in practice. Its technology is backed by peer-reviewed research and supported through comprehensive provider training and U.S.-based service. Used in leading medical centers and high-performance environments, it delivers consistency that clinicians can depend on and patients can feel.
Discover how SoftWave Therapy can expand your treatment capabilities and strengthen clinical outcomes. Schedule a Demo or Become a SoftWave Provider today.